

TIMELINE

Timeline

InnoPack will launch the Awards in China during its show in 2024. A ceremony will be held to recognise innovative pharmaceutical packaging products, solutions and technologies during the show dates onsite. Celebrating the latest industry developments, the Awards aim to provide inspiration to packaging companies and drive innovation within the pharmaceutical packaging industry.
Who Can Apply?
| • Packaging or Drug Delivery Device Suppliers | • Pharmaceutical, Veterinary or Biopharmaceutical Companies |
| Packaging or drug delivery device suppliers with innovative packaging solutions and/or products for pharmaceutical or biopharmaceutical drug products. Entries must demonstrate clear innovation in ensuring drug quality, improving patient-centricity and adherence, or supporting sustainable development. | Pharmaceutical, veterinary or biopharmaceutical companies with innovative packaging or drug delivery devices for their solutions. These companies may apply in cooperation with the packaging providers. |
| NB: Entries will only be considered if the product has been launched in the past 5 years | |
Categories & Criteria





This will be awarded to a product that helps to optimize the performance of pharmaceuticals with an innovative packaging solution or drug delivery device.
- Does the product answer a previously unmet need?
- Is it the first of its kind on the market?
- Does the product achieve and improve the drug’s therapeutic effect?
This will be awarded to a product or company that has made an innovative change in the practical application of the medical aesthetic field (resulting in smaller wounds, no pain, or the use of new technologies, etc.), and has achieved an excellent balance between drug delivery efficiency and compliance.
- Does the product have innovative points in the actual use?
- Does the product improve efficiency and ensure safety in actual practice?
- Is the product executable?
This award will be given to a product or company that helps to improve patient compliance through innovative or improved packaging solutions (including packaging materials, drug delivery forms and intelligent interactions, etc.)
- Does the product have innovative points in compliance?
- Does the product achieve the actual treatment compliance improvement among the users?
- Is the product or solution executable?
This will be awarded to innovative product or company that achieve safe and effective drug delivery in ophthalmic, parenteral, oral, respiratory, and other drug delivery solutions.
- Does the product satisfy an unmet demand or introduce new ideas, technologies to the market?
- Does it improve the safety of use by patients or healthcare workers?
- Is the product executable?
This will be awarded to a product or company that optimize pharma storage and transportation space efficiency through packaging innovation or eco-friendly pharma packaging material and design.
- Does the product bring good protection for drug during storage and transportation?
- Does the product use green or recycling material or design?
Startups are often at different stages of development, so the entries that are still in the concept stage or not commercialised are accepted. This will be awarded to a product or a company that can lead the future of packaging by innovating, growing or sustainably contributing to the pharmaceutical industry.
- Explain the innovation of the product or solution
- Judge if the product has an approved patent or a pending patent.
- Evidence that the product or solution have created a new market opportunity (not limited to profit only).
- Does the product use green or recycling material or design?
How to Define the Winner?
- Each category will have 1 winner only and maximum 2 runners-up.
- The Jury will first select 25 nominees out of all entries for further vote and score.
- Viewers will vote on official show WeChat and Website. Top 5 entrees of the vote will get the score of 100, 95, 85, 70 and 50.
- Each member in the Jury will give a score out of 100 and takes the average as final Jury score.
- Viewers vote accounts for 50% of final score and Jury votes for 50% of final score.
Promotion
For all nominees
- Pre-show: nominees’ company logo and introduction with product pictures promoted via official channels including website, WeChat, EDM, etc
- On-site: nominees’ company logo will be printed on Hall N5 floorplan in the on-site Show Guide publication
Winners' Prize:Extra Promotional Package
- On-site: winner’s company logo and products will be posted by official WeChat on first day of the show
- Post-show: winners’ company article will be posted on official WeChat.
The Winners in 2022
「Functionality」
(2).png)
Target medicine types/ users:
•Biological drugs with large volume and high viscosity
•Subcutaneous(SC) self-administration
Purpose of the R&D/ design/ improvement:
With a patient-centric focus, SmartDose™ Drug Delivery Platform makes treatment more patient-friendly with home administration. The pre-programmable, easy-to-use wearable injectors that can hold larger volumes of drugs and infuse them SC over a long period of time are becoming a viable way to achieve self-administration, boosting user confidence, maximizing comfort for a superior user experience and then allowing the patient to continue an active lifestyle.
Product introduction:
Driven by research, SmartDose™ Drug Delivery Platform provides an optimized user experience through an intuitive interface, a streamlined workflow, and ergonomics for various disease state applications. The platform is offered for 3.5 mL and 10 mL doses. Delivery rates are pre-programable, and high viscosities can be handled. Integrated technology is under development to enable wireless communication of device data and status to support connected health and patient engagement platforms.

Target medicine types/ users :
Adella Unidose System is developed for drug use, such as the treatment of migraines, epilepsy and analgesia and other chronic central nervous system diseases, as well as for treating hypoglycemia and to counter an Opioid overuse.
Purpose of the R&D/ design/ improvement:
One-step nasal liquid drug delivery device designed for Asian patients
Product introduction:
Adella Unidose System is designed for Asian patients, ready-to-use, one-step nasal liquid drug delivery device which delivers a precise, single dose quickly, easily and reliably. This patented system can be applied for chronic CNS conditions such as migraine, epilepsy and pain relief as well as for drugs to treat hypoglycemia and to counter an Opioid overuse.

Target medicine types/ users:
Beauty, skin care, anti-oxidation
Purpose of the R&D/ design/ improvement:
Environmental protection, protect fragile formula
Product introduction:
Environmentally friendly recyclable packaging can effectively protect fragile formulas and improve shelf life and consumer experience. Berry Ace group introduces a full set of AIRFREE® production line from Europe, which provides each brand with a packaging material composed of "6 layers Co-Ex bottle (Bag-in-bottle technology with EVOH as barrier " and "Full plastic ECO-pump”, which will help our customers positioned as more competitive in the market.
The product can be applied to viscosity from liquid to thick creams with consistent dosing.
Berry Global Group, Inc.
Target medicine types/ users:
Insulin、GLP-1、Protein Medicine、Somatotropic Hormone、Uretinoin、Monoclonal antibody
Purpose of the R&D/ design/ improvement:
Easy dose setting press button design - ensuring high patient comfort, ergonomically proven and tested
Product introduction:
It is an automated disposable injection device for pre-filled syringe suitable for all patient groups.The device is a push-on-skin activation,being convenient,and preferred by patients.Audible confirmation“clicks”after insertion,end of injection and needle protection increase patient confidence.
Gold Winner

1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Product:
Antimicrobial ophthalmic multidose eye dropper
Company:
Berry Global Group, Inc.
Silver Winner
1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Product:
RayDylyo Push Fit Cap
Company:
ARaymondlife SASU
Silver Winner
1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Product:
NovaGuard® SA Pro system
Company:
West Pharmaceutical Packaging (China) Company Ltd.
「Market Potential」
Gold Winner

1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Product:
Ophthalmic Bottle
Company:
Gerresheimer China
Silver Winner
1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
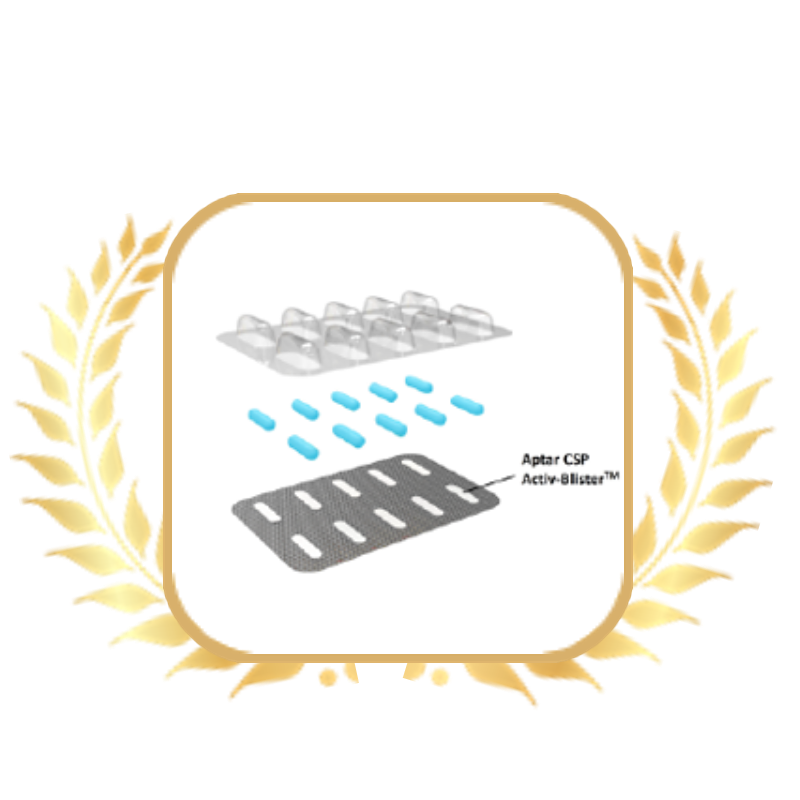
Product:
Activ-Blister™ Solutions
Company:
Aptar CSP Technologies
Silver Winner

1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Product:
Smart Auto Injector
Company:
Jiaxing Summed Medtech Co., Ltd.
「Patient Adherence」
Gold Winner
1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Product:
HeroTracker® Sense
Company:
Aptar Pharma
Silver Winner

1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Product:
Auto-Injector
Company:
Suzhou Healthy Tree Medical Technology Co., Ltd
Silver Winner

1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Product:
Ypsomate Zero autoinjector
Company:
Ypsomate AG
「Cost effective」
Gold Winner

1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Product:
FlexPack
Company:
Körber Pharma (Shanghai) Co., Ltd.
Silver Winner

1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Product:
Antimicrobial ophthalmic multidose eye dropper
Company:
Berry Global Group, Inc.
Silver Winner

1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Product:
Needle-Trap
Company:
Schreiner MediPharm
「Eco-friendly」
Gold Winner
1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Product:
kpNext™ RB5 blister film
Company:
Kloeckner Pentaplast (Suzhou) Specialty Materials Co., Ltd.
Silver Winner

1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Product:
MDI Plasma coated aluminium canister
Company:
Anomatic Suzhou Metal Packaging Co.,Ltd
Silver Winner

1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Product:
Forest Film
Company:
UPM Raflatac
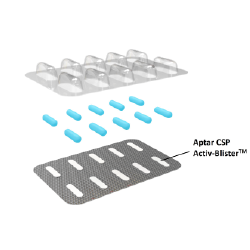
由于水分、氧气和杂质渗入包装,越来越多的制药合作伙伴面临口服固体制剂稳定性相关的挑战。
Aptar CSP Technologies的 Activ-Blister™提供了一种新的解决方案,特别是针对口服固体制剂和胶囊型干粉吸入装置,以延长产品货架期并确保其效价。我们的定制解决方案以对患者友好的包装方式无缝集成到客户新的或现有的包装产线中,为每个泡罩内部创造特定的微环境,以满足药物长期保存和稳定性的要求,并保持产品效价。配备Activ-Blister™解决方案的泡罩可以吸附特定量的水分、清除氧气、异味、有机挥发物或提供活性组合保护。
Activ-Blister™ 解决方案采用拥有专利技术的热合工艺将定制配方的 Aptar CSP Technologies 3-Phase Activ-Polymer™(专利技术)薄膜嵌入到每个单独的泡罩中。热合工艺将该技术牢固地固定到铝箔上,而无需使用粘合剂。该方法极具成本效益,避免了残留溶剂可能释放气味并与药品发生反应。该薄膜可以定制为任何尺寸的片剂或胶囊,确保该技术可以在不影响药物的情况下进行操作。该技术可应用于穿透式泡罩、剥离/挤压式铝塑泡罩、冷成型铝箔、高阻隔薄膜、PVdC 涂层或 EVOH 层压薄膜、Alu/Alu 泡罩和高阻隔热成型。

kpNext™ RB5能够完美替代传统的阻隔类泡罩包装薄膜,助力医药行业实现可持续发展,同时该产品还具备相当于90g/m² 的PVdC材料的高阻湿性能。kpNext™ RB5适用于5号回收标识并能助力客户改善碳足迹。
作为一款非PVC类的泡罩包装薄膜,kpNext™ RB5能够与现有生产线完全兼容,无需额外的设备投资,且加工使用过程中的性能表现与PVC类产品相当。
Berry Global Group, Inc.

Forest Film™是一款源自于受可持续管理森林的100%纯木质基薄膜标签解决方案,全球包装行业正在积极应对使用一次性塑料所带来的问题,并关注包装材料的可循环能力。用可再生材料代替传统化石原材料的需求日益增长。
作为UPM的一部分,我们致力于超越化石材料,通过探索和创新开创一个绿色的未来;并坚持寻找更加智能、更具可持续性的日用品材料。我们支持循环经济,并签署了由艾伦 · 麦克阿瑟基金会发起的《 新塑料经济全球承诺书》。
作为第一个向市场引入 Forest Film™ - 新型木质基薄膜标签材料生产商,我们在可持续标签领域迈出了领先的一步,满足了客户以可再生材料取代传统化石原材料的需求。该产品与 UPM 生物燃料事业部合作开发,采用了UPM BioVerno 石脑油,是一款源自于受可持续管理森林的 100% 纯木质基薄膜标签解决方案,为制浆过程中的残留物赋予了新生。
经过验证, Forest Film™ 系列产品可以节省温室气体的排放。相较于采用石油基的传统薄膜材料产品, Forest Film™ 与传统化石基薄膜产品在性能、外观及回收能力方面毫无差别,可以根据不同的应用搭配不同功能的胶水,实现专业医药包装的安全保障同时促进可持续目标推进。

随着预灌封自动注射器逐渐普及,药物输送设备行业急需面对世界上最大挑战之一:塑料使用及其对环境的影响。我们会即刻采取行动并认真对待行业和环境赋予我们的责任!
Ypsomed建立了一个完整可持续发展的良性循环。我们不仅仅在产品制造环节大幅降低碳排放,还致力于持续减少在原材料,生产,回收,再利用环节产生的碳排放量,从而使产品的整个生命周期形成一个减碳闭环。
Ypsomed对 YpsoMate两步式自动注射器进行了生命周期评估,得到了其整个生命周期包括原材料、制造和最终处置的环境成本定量数据。生命周期分析为减少 YpsoMate 碳排放提供了坚实的基础,并将工程工作导向最关键的三个环节:
1.在选定的设备组件和包装中使用替代聚合物
2.关闭设备组件和设备子组件的包装循环
3.在开发过程中实施回收设计
YpsoMate – the 2-step autoinjector
格雷斯海姆E系列滴眼剂瓶,符合美国FDA对滴眼剂瓶的最新要求——既拧开后防盗环需卡在瓶子上而不能掉落。同时我们为低粘度眼科药物开发了一种新型内塞,防止给药过程中液滴不受控制地从瓶子中流出,以确保药物均匀滴落直至最后一滴,使用起来会比以前更为便捷。
Eyedropper
Gerresheimer AG

芬欧蓝泰RP Cryo 超低温医药标签胶黏剂耐受苛刻的低温条件,可用于低至-150至-196摄氏度的液氮储存,特别适用于低温冻存容器。即使面临潮湿和寒冷表面,标签仍然具有良好的粘附力同时配合合适的标签面材时,标签信息打印效果清晰;经过专门设计,在搭配合适的标签面材,可耐受多次冻融循环:即使在恶劣环境的储存和取放后仍然保持完好无损且不卷翘,不会吸水受潮,即使解冻后也不发生撕裂。
Ultralow Temperature Pharmaceutical Label Adhesive

西氏的NovaGuard® SA Pro安全系统旨在保护医护人员和患者免受意外针刺伤害。NovaGuard® SA Pro安全系统具有防止操作过程中预激活的设计,仅需单手即可操作。该系统与ISO 0.5mL标准和1mL细长玻璃注射器(带针)兼容,其透明的外观便于药物检查和注射。此外,该装置的设计有助于玻璃预填充注射器的轻松组装,能够实现现有灌装设备轻松改装。
产品优势包括:
• 具有防止操作过程中预激活的设计
• 能轻松激活,便于终端用户使用
• 透明度高,便于药品检查和标签设置
• 易于在低速和高速灌装线上组装
• 与标准推杆兼容
• 防篡改功能,可防止硬护帽再次装上针头,符合EU指令2010/32/EU
West Pharmaceutical Services

阿普塔医药PremiumCoat®平台(包含产品和服务)为注射剂瓶和预灌封注射器提供溴化配方的ETFE覆膜瓶塞和活塞解决方案,以及为客户定制一整套关键数据和服务包,帮助客户加速药物研发。
PremiumCoat®解决方案可用于13mm和 20mm的注射剂瓶,1mlL预灌封注射器,以及正在商业化的1-3mL预灌封注射器。
阿普塔医药研发了一系列先进的检测评价方法来测试和体现PremiumCoat®的性能。阿普塔医药服务汇集了服务公司(阿普塔医药子公司)的专长,为客户提供端到端的服务,以加快产品研发并降低研发过程中的风险。
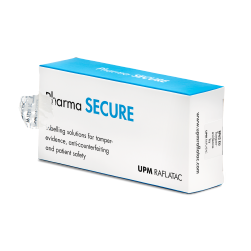
在制药市场中,假药问题日益严重。能够显示药品是否被开启过的安全封口标签可以帮助患者确认药品的真假和可靠性。我们的安全标签解决方案可以帮助您遵从法律法规要求并提升患者安全性。具体包括:
1.封口标签,移除后,会使包装或标签发生明显的、不可逆转的损坏或更改 - 不会增加包装的打开难度。
2.封口标签的功能包括纤维撕裂、VOID 效果、易碎纸张和薄膜以及全息图防伪。
3.存在验证解决方案,可以更轻松地确保防开启标签存在并正确粘贴在包装上。

1.可根据剂量要求进行客户定制化设计,有0.35ml,0.5ml,1ml,2.25ml。
2.现有的一次性注射器,设计原理都非常复杂,导致零部件繁多,普遍在15个以上,因此可靠性不高,装配难度大,而且产品成本高;我们提供了一种结构简单,零部件数量显著减少,且各注射步骤可靠性高的一次性自动注射器。
3.注射笔外壳体采用凸条设计,在满足防滑的同时不会造成手指或手掌的拿捏不适感。
4.采用新型的自动注射器触发装置,增加限位,避免二次触发,采用模块化配置,易于装配与使用,安装容错率较高,即便安装错误也可以快速进行替换,降低装配成本。
Suzhou Jiashu Medical Technology Co., Ltd.

1.通过在瓶盖中集成干燥剂,达到防潮作用,防止产品失效。
2.通过瓶与盖的配合达到密封效果,阻隔环境中的水气进入包装,延长包装的时效,同时具有反复密封作用,客户使用过程中依然能有效保护产品。
3.通过盖子的特殊设计,达到防儿童开启效果,兼具防盗功能。
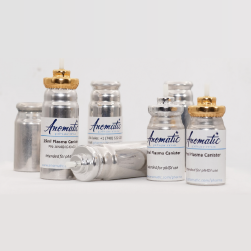
1. 涂层工艺是用高纯度无溶剂气体。
2. 超纯单体,低碳环保。
3. 涂层材料使铝罐表面产生了一种低表面能具有疏水性,极大的提高了罐装物药物的传递率。

NovaPure®活塞的设计开发充分运用了“质量源于设计”(Quality by Design, QbD)这一原理。根据客户和终端用户的需求,西氏制定了全面的产品质量目标(QTPP)。始终将终端患者的需求放在首位,关键质量属性(CQA)的建立有助于确保整个生命周期内药品的质量、安全性和有效性。
NovaPure®活塞优化了启动力和滑动力,严格的设计质量标准减小了产品部件间的尺寸差异,适配不同注射体积和更高粘度范围的自动注射器的药物注射。
所有NovaPure®组件均符合西氏的严格质量标准:制药级清洗、灭菌、FluroTec®阻隔膜,以及完整的West Envision™自动外观检查。
West Pharmaceutical Services

1.创新性的压盖,匹配所有符合ISO标准的胶塞和西林瓶。
2.通过包装密封完整性验证(-80°C,常温,40°C)。
3.预装胶塞,免洗免灭。
4.无菌区同步加塞与压盖。
5.可手工压盖,无需任何工具。
6.无需轧盖工序,降低设备、公用设施、无菌区、人员、验证等投入。
7.无粘塞,不跳塞。
8.无铝屑,极大减少颗粒物。
9.可实现盖身喷码和贴标。
10.无金属边缘割破手套风险。

1. 该产品采用分体设计,盖子和管子是分开的且盖子及干燥剂一体,简化了客户端的流程,即不需要单独在管内投放袋装干燥剂或筒状干燥剂,避免了小孩误食和干燥剂污染产品。
2. 该包装由于是分体,很轻松地实现防盗环的功能,避免人为的开启后来投诉产品已被使用过的可能。
3. 设计简约美观,符合人体工学,盖子带水滴曲面的设计,可以轻易的单手开启,开启后盖子与管子相连,防止忘记合盖产品受潮。
4.该产品的最大的特点为:吸湿相关的功能优秀,对测试条的起到很好的保护。
提交企业:SANNER
 院外自我注射,操作简便,给药精准,高度智能、数据可追溯,提高病患依从性。适用范围广泛,可满足高粘度、大剂量药物注射要求(1mL, 2.25mL)。驱动装置重复使用,减少浪费,节约社会资源。
院外自我注射,操作简便,给药精准,高度智能、数据可追溯,提高病患依从性。适用范围广泛,可满足高粘度、大剂量药物注射要求(1mL, 2.25mL)。驱动装置重复使用,减少浪费,节约社会资源。

-2.png)
Target medicine types/ users:
Eye dropper/suitable to all users, especially friendly to senior, pediatrics and veterinary users.
Purpose of the R&D/ design/ improvement:
More accurate medication and improve medication compliance.
Product introduction:
Our new Risdrop™ product was carefully designed for the ophthalmic market, creating an innovative user-independent eye-dropper with a unique nozzle technology. The same size drop every time, helping reduce the risk of over dosage. The nozzle features a restricted flow channel insert allowing for a uniform drop size (variance +/-15%) regardless of container inclination and irrespective of squeeze force. The package helps the delivery of the drop to the right spot and is easy-to-hold and friendly to senior, pediatrics and veterinary users.
RISDROP™- User Independent Eye Dropper with Consistent Drug Dispensing
Berry Global Group, Inc.
.png)
Target medicine types/ users :
Aesthetic/mRNA vaccines/IV Infusion
Purpose of the R&D/ design/ improvement:
Innovation Polymer Syringe to meet high challenge drugs requirement.
Product introduction:
SCHOTT TOPPAC® is made of 100% high purity materials of Cyclic olefin copolymer (COC) which had good resistance to damage, transparency comparable to glass, and excellent barrier properties, no ion or metal released and no PH shift, highly suitable for biologics and low protein adsorption.
SCHOTT TOPPAC® Pre-Filled Syringe

Target medicine types/ users:
Applications for pills and capsules
* Over-the-counter (OTC)
* Ethicals
* Generics
* Nutraceuticals
* Veterinary medicine
Purpose of the R&D/ design/ improvement:
Designed to be Recyclable
Product introduction:
kpNext™ R1 is a PET mono rigid film that is designed to be fully recyclable within the RIC #1 stream. It is an alternative to vinyl blister films that will meet or exceed all your sustainability goals and work within your current manufacturing setup. Pharmaceutical companies and converters can utilize kpNext™ R1 on their existing form, fill and seal lines with no loss of line speed or a need to retool.

Target medicine types/ users:
Target medicines are asthma adn COPD drugs such as Budesonide Formoterol.
Purpose of the R&D/ design/ improvement:
PowdAir Plus has been designed to be a cost effective dry powder inhaler, made from four plastic components, and with no metal components. This minimises manufacturing and assembly costs. The sleek design ensures the device is easy to use for the patient.
Product introduction:
In developing markets, where demand for respiratory treatments is growing rapidly, there’s an increasing need to offer more choice to patients who use a dry powder inhaler (DPI).PowdAir Plus is a patented capsule-based DPI, ideal for these markets because of its advanced simplicity, ease of use and affordability.Most other DPIs have been designed for developed markets and incorporate multiple components and complex mechanics. In contrast, PowdAir Plus is a complete unit with no metal or separate parts. Neat and compact, its novel all-plastic, four-component design minimises manufacturing assembly and production costs, while improving the device’s resilience to frequent use.The sleek design of PowdAir Plus makes this inhaler very easy to use. It’s highly portable, and delivers an effective dose of medication with each capsule. Compatible with HPMC capsules in size 3, it can be used with any dry powder medicines.A particularly useful design feature is the way the device opens the capsule as the tray closes. Patients don’t need to pierce the capsule themselves, so using the inhaler is simpler, and theres no risk of getting the wrong dose.


Target medicine types/ users:
Compitable with dial-and-dose disposable pen(Unopen)
Purpose of the R&D/ design/ improvement:
Transforming the proven dial-and-dose pen platform into a connected
Product introduction:
Smart Pilot could Transform the proven dial-and-dose pen platform into a connected system though a single click (and clear visual feedback). Real-time tracking function not only provide dosing record for patient but also doctors and relation for better adherence and cure.
Smart Pilot™ -An Intelligent Device Designed for Mature Injection Pen Platforms
YPSOMED AG
.jpg)
Target medicine types/ users:
Applicable for sterile filling with vials
Purpose of the R&D/ design/ improvement:
Sustainable solution to treating the internal surfaces of pMDI canisters, and helping combat drug degradation/drug adhesion which are common problems within a pMDI, thus ensuring the patient receives the correct dose each and every time.
Product introduction:
The innovated elastomer sealing set can be widely used for serum products, Lyophilization, and IV solutions without filling in the different lines. This packing system can replace the general packing with vials, rubber stoppers and Aluminum seals, and simplifies production process and secured the products.
New Elastomer Sealing Solution includes special designed vials, plastic/elastomer sealing set, improving the current packing system without general metal material. In this case, no metal particles will be created. And for Lyophilization products, capping can be proceeded in Lyophilization machine. So that production process will be more efficiency and decreasing the cost.
Target medicine types/ users:
Vaccine and psychotropic drugs/drug manufacturers
Purpose of the R&D/ design/ improvement:
Improve the use experience and treatment effect, improve compliance
Product introduction:
Single dose trigger nasal spray device adopts a new structure with a spring as the driving force of drug delivery. Different users only need to press lightly to trigger the release force of the spring, which can push the storage component to move and make the medium in the storage component spray to the nasal cavity through the nozzle component. It can be applied to different populations and is easy for patients to operate, and ensures that different types of patients can deliver the same dose at the same injection speed, thus improving the use experience, treatment effect and compliance.
Aerosol – Single Dose Trigger Nasal Spray Device
Target medicine types/ users:
Pharmaceutical packaging products
Purpose of the R&D/ design/ improvement:
Pharmaceutical packaging that requires higher storage time and is more sensitive to moisture
Product introduction:
This product is optimized in the structure of cold forming process aluminum by processing and compounding of key moisture-absorbing materials. The structure is OPA/AL/polyolefin moisture-absorbing layer/PVC, which takes into account the overall cost of moisture absorption, especially the cost of PTP. PTP can still use ordinary PTP with registration number. It can greatly reduce the cost of use and R&D for customers. At the same time, our company adopts multi-layer co-extrusion technology in the polyolefin moisture-absorbing layer and adds a barrier layer and slow-release layer. We can design and adjust the formula according to the customers’ requirements of the moisture absorption curve (The relationship between time and moisture absorption) to achieve the packaging effect matching with the drug compatibility.
Moisture Control Functional Cold Forming Process Aluminum Packaging Products Mainly for The Type of Drugs

Target medicine types/ users:
Solid preparations, injections, ointments, etc.
Purpose of the R&D/ design/ improvement:
Long-term improvement of efficiency and continuous cost saving.
Product introduction:
The project improved brand consistency management standards, increased management and production efficiency, reduced production and transportation costs, and achieved the long-term sustainable goal of effectively improving efficiency and saving costs.

Target medicine types/ users:
an oral liquid preparation package for accurate quantitative
Purpose of the R&D/ design/ improvement:
accurate quantitative and non-cleaning
Product introduction:
The utility model relates to an oral liquid preparation package for accurate quantitative and non cleaning. The storage part of the taking measuring cup is used to store the poured liquid preparation, and a scale line is arranged inside to accurately measure and quantify the taking amount. The taking part extends out of the storage part, and the liquid preparation in the storage part is guided into the patient's mouth from the accurately measured taking part, which makes it more convenient to take and avoids spilling the liquid preparation during taking. The built-in taking part does not contact the preparation after use, and is isolated from the air after locking the bottle cap, which does not affect the drug quality. At the same time, it reduces the inconvenience of patients' use and cleaning caused by external measuring cup.
The Utility Model Relates to An Oral Liquid Preparation Package for Accurate Quantitative and Non-Cleaning.
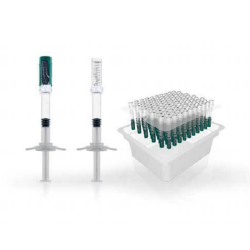
The Entries in 2022

(2).png)
Target medicine types/ users:
•Biological drugs with large volume and high viscosity
•Subcutaneous(SC) self-administration
Purpose of the R&D/ design/ improvement:
With a patient-centric focus, SmartDose™ Drug Delivery Platform makes treatment more patient-friendly with home administration. The pre-programmable, easy-to-use wearable injectors that can hold larger volumes of drugs and infuse them SC over a long period of time are becoming a viable way to achieve self-administration, boosting user confidence, maximizing comfort for a superior user experience and then allowing the patient to continue an active lifestyle.
Product introduction:
Driven by research, SmartDose™ Drug Delivery Platform provides an optimized user experience through an intuitive interface, a streamlined workflow, and ergonomics for various disease state applications. The platform is offered for 3.5 mL and 10 mL doses. Delivery rates are pre-programable, and high viscosities can be handled. Integrated technology is under development to enable wireless communication of device data and status to support connected health and patient engagement platforms.

Target medicine types/ users :
Adella Unidose System is developed for drug use, such as the treatment of migraines, epilepsy and analgesia and other chronic central nervous system diseases, as well as for treating hypoglycemia and to counter an Opioid overuse.
Purpose of the R&D/ design/ improvement:
One-step nasal liquid drug delivery device designed for Asian patients
Product introduction:
Adella Unidose System is designed for Asian patients, ready-to-use, one-step nasal liquid drug delivery device which delivers a precise, single dose quickly, easily and reliably. This patented system can be applied for chronic CNS conditions such as migraine, epilepsy and pain relief as well as for drugs to treat hypoglycemia and to counter an Opioid overuse.

Target medicine types/ users:
Beauty, skin care, anti-oxidation
Purpose of the R&D/ design/ improvement:
Environmental protection, protect fragile formula
Product introduction:
Environmentally friendly recyclable packaging can effectively protect fragile formulas and improve shelf life and consumer experience. Berry Ace group introduces a full set of AIRFREE® production line from Europe, which provides each brand with a packaging material composed of "6 layers Co-Ex bottle (Bag-in-bottle technology with EVOH as barrier " and "Full plastic ECO-pump”, which will help our customers positioned as more competitive in the market.
The product can be applied to viscosity from liquid to thick creams with consistent dosing.
Berry Global Group, Inc.

Target medicine types/ users:
Insulin、GLP-1、Protein Medicine、Somatotropic Hormone、Uretinoin、Monoclonal antibody
Purpose of the R&D/ design/ improvement:
Easy dose setting press button design - ensuring high patient comfort, ergonomically proven and tested
Product introduction:
It is an automated disposable injection device for pre-filled syringe suitable for all patient groups.The device is a push-on-skin activation,being convenient,and preferred by patients.Audible confirmation“clicks”after insertion,end of injection and needle protection increase patient confidence.
Gx InnoSafe®是一种被动集成安全系统,可防止意外刺伤并可避免重复使用。其设计简单且经过测试,可避免不当操作或无意中触动安全注射器。安全系统可完全在格雷斯海姆的预灌装流程中自动组装,并且注射器已被包装、密封和消毒好。
Gx InnoSafe®采用符合ISO公认标准的蜂巢包装方式,与普通针头注射器相比,其在灌装药物时无需调整灌装设备,所有灌装厂(制药公司)不需要进行任何投资,即可在现有注射器灌装线上轻松灌装Gx InnoSafe® 安全注射器。
Gerresheimer AG

FlexPack最大的创新,是使用统一规格的全纸内衬根据需求兼容包装不同尺寸的容器,且确保每个容器之间不碰触、获得良好地保护。
高度的灵活性及其效率,使得更少的内衬与小盒规格变化就能满足多种尺寸产品的包装需求。
例如:包装不同大小的西林瓶,我们只需要1种尺寸的小盒和内衬,内衬的产品置入处根据西林瓶的尺寸设计不同位置的穿孔线。当需要放置大尺寸西林瓶时,只需按大尺寸处的穿孔线置入西林瓶;放置小尺寸西林瓶时,按小尺寸处的穿孔线置入。
此前,当使用全纸包装时,不同尺寸的西林瓶需要不同的内衬。采用FlexPack后,能使生产变化和成本保持在极低水平,同时模块化的设计意味着可以大大降低工艺和存储成本。
Körber Pharma
HeroTracker®Sense旨在帮助世界各地患有慢性呼吸系统疾病(如哮喘、慢性阻塞性肺病、囊性纤维化和其他由COVID-19引起的呼吸系统症状)的患者改善生活,跟踪他们的定量吸入气雾剂(MDI)药物使用情况,并提高他们对药物的用药依从性。HeroTracker® Sense 是新一代MDI附加连接装置,旨在提高患者吸入技巧和依从性。
主要特点 :
将HeroTracker®Sense连接到定量吸入气雾剂(MDI)的药罐上能实现多种功能,帮助哮喘和慢性阻塞性肺病患者以更知情和依从的方式吸入药物。装置中的传感器能够产生和提供信息,例如吸气与按压动作的协调、吸气流速和吸气持续时间。该应用的其他功能还包括日期和时间标签,以提示患者应该何时服药,以及温度和湿度等环境监测警报。
与符合HIPAA和GDPR标准的阿普塔医药子公司Cohero Health的BreatheSmart®Connect Portal相连, HeroTracker®Sense为医疗保健提供者(HCPs)提供关于患者训练、使用培训和患者表现的有价值的分析和洞察,同时患者可以通过BreatheSmart®应用程序监测自己的用药依从情况。
HeroTracker®Sense智能连接装置还可重复使用,一旦患者用完了MDI 药物,便可移除该附加连接装置并将其附加到新的MDI 上。替换操作可多次进行。
HeroTracker®Sense为制药合作伙伴提供了一个简单的连接装置,是一种接触少、影响深远的解决方案,将帮助患者提升依从性并改善患者健康和生活质量。
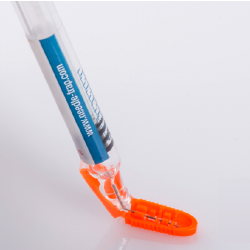
Needle-Trap是一款标签和安全装置二合一的独特设计。创新的标签设计可以让用户在注射药物后把针尖快速收纳在接连的 ‘捕集器’ 配件里,防止针刺扎伤引起感染。Needle-Trap易于使用,无需变更注射方式;也可以在生产药物的贴标过程中自动加入销毁针尖的配件。
作为合符医护人员和医药工业要求的产品,Needle-Trap既可以在医疗操作中提供安全便捷,同时让医药生产商在成本效益、灵活和可靠的流程受益。
由于Needle-Trap小巧的设计,让它的可持续性发挥得淋漓尽致。塑料的 ‘捕集器’ 含有高达50%的再研磨材料。和其他传统的针尖防护系统相比,它包含较少的塑料、没有金属。由于设计极为小巧,无需更改药物的外包装,也就是说无论在运输、仓储、冷冻和弃置的环节,可以达至空间最小化,从而减低环境足迹。

由于水分、氧气和杂质渗入包装,越来越多的制药合作伙伴面临口服固体制剂稳定性相关的挑战。
Aptar CSP Technologies的 Activ-Blister™提供了一种新的解决方案,特别是针对口服固体制剂和胶囊型干粉吸入装置,以延长产品货架期并确保其效价。我们的定制解决方案以对患者友好的包装方式无缝集成到客户新的或现有的包装产线中,为每个泡罩内部创造特定的微环境,以满足药物长期保存和稳定性的要求,并保持产品效价。配备Activ-Blister™解决方案的泡罩可以吸附特定量的水分、清除氧气、异味、有机挥发物或提供活性组合保护。
Activ-Blister™ 解决方案采用拥有专利技术的热合工艺将定制配方的 Aptar CSP Technologies 3-Phase Activ-Polymer™(专利技术)薄膜嵌入到每个单独的泡罩中。热合工艺将该技术牢固地固定到铝箔上,而无需使用粘合剂。该方法极具成本效益,避免了残留溶剂可能释放气味并与药品发生反应。该薄膜可以定制为任何尺寸的片剂或胶囊,确保该技术可以在不影响药物的情况下进行操作。该技术可应用于穿透式泡罩、剥离/挤压式铝塑泡罩、冷成型铝箔、高阻隔薄膜、PVdC 涂层或 EVOH 层压薄膜、Alu/Alu 泡罩和高阻隔热成型。
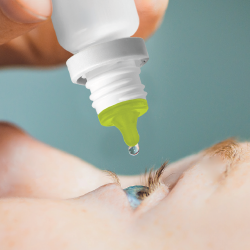
1.根据临床研究(Nentwich et al, Br J Ophthalmol 91(10): 1265-68)显示,85%的污染都发生在滴嘴尖端,而这款产品对于滴嘴尖端的抗菌矿物质保护作用经过了验证和认证。
2.确保患者在每一次使用多剂量滴眼液瓶时都是卫生安全的。
3.与单剂量滴眼液产品相比,基于一个月疗程用量,可减少16倍的塑料废弃物。
4.不改变患者治疗方式。
5.完全兼容、稳定且生物相容,同时经过认证的无刺激性和无细胞毒性技术(ISO10993-2010)。
6.无需对包装设计或现有制造/灌装工艺进行修改。
7.通过Pylote公司的矿物抗菌技术,这种新型多剂量滴眼液瓶已被证明对3型腺病毒(结膜炎)、大肠杆菌和金黄色葡萄球菌有效。
Antibacterial Eyedropper
Berry Global Group, Inc.

kpNext™ RB5能够完美替代传统的阻隔类泡罩包装薄膜,助力医药行业实现可持续发展,同时该产品还具备相当于90g/m² 的PVdC材料的高阻湿性能。kpNext™ RB5适用于5号回收标识并能助力客户改善碳足迹。
作为一款非PVC类的泡罩包装薄膜,kpNext™ RB5能够与现有生产线完全兼容,无需额外的设备投资,且加工使用过程中的性能表现与PVC类产品相当。
Berry Global Group, Inc.

Forest Film™是一款源自于受可持续管理森林的100%纯木质基薄膜标签解决方案,全球包装行业正在积极应对使用一次性塑料所带来的问题,并关注包装材料的可循环能力。用可再生材料代替传统化石原材料的需求日益增长。
作为UPM的一部分,我们致力于超越化石材料,通过探索和创新开创一个绿色的未来;并坚持寻找更加智能、更具可持续性的日用品材料。我们支持循环经济,并签署了由艾伦 · 麦克阿瑟基金会发起的《 新塑料经济全球承诺书》。
作为第一个向市场引入 Forest Film™ - 新型木质基薄膜标签材料生产商,我们在可持续标签领域迈出了领先的一步,满足了客户以可再生材料取代传统化石原材料的需求。该产品与 UPM 生物燃料事业部合作开发,采用了UPM BioVerno 石脑油,是一款源自于受可持续管理森林的 100% 纯木质基薄膜标签解决方案,为制浆过程中的残留物赋予了新生。
经过验证, Forest Film™ 系列产品可以节省温室气体的排放。相较于采用石油基的传统薄膜材料产品, Forest Film™ 与传统化石基薄膜产品在性能、外观及回收能力方面毫无差别,可以根据不同的应用搭配不同功能的胶水,实现专业医药包装的安全保障同时促进可持续目标推进。

随着预灌封自动注射器逐渐普及,药物输送设备行业急需面对世界上最大挑战之一:塑料使用及其对环境的影响。我们会即刻采取行动并认真对待行业和环境赋予我们的责任!
Ypsomed建立了一个完整可持续发展的良性循环。我们不仅仅在产品制造环节大幅降低碳排放,还致力于持续减少在原材料,生产,回收,再利用环节产生的碳排放量,从而使产品的整个生命周期形成一个减碳闭环。
Ypsomed对 YpsoMate两步式自动注射器进行了生命周期评估,得到了其整个生命周期包括原材料、制造和最终处置的环境成本定量数据。生命周期分析为减少 YpsoMate 碳排放提供了坚实的基础,并将工程工作导向最关键的三个环节:
1.在选定的设备组件和包装中使用替代聚合物
2.关闭设备组件和设备子组件的包装循环
3.在开发过程中实施回收设计
YpsoMate – the 2-step autoinjector

格雷斯海姆E系列滴眼剂瓶,符合美国FDA对滴眼剂瓶的最新要求——既拧开后防盗环需卡在瓶子上而不能掉落。同时我们为低粘度眼科药物开发了一种新型内塞,防止给药过程中液滴不受控制地从瓶子中流出,以确保药物均匀滴落直至最后一滴,使用起来会比以前更为便捷。
Eyedropper
Gerresheimer AG

芬欧蓝泰RP Cryo 超低温医药标签胶黏剂耐受苛刻的低温条件,可用于低至-150至-196摄氏度的液氮储存,特别适用于低温冻存容器。即使面临潮湿和寒冷表面,标签仍然具有良好的粘附力同时配合合适的标签面材时,标签信息打印效果清晰;经过专门设计,在搭配合适的标签面材,可耐受多次冻融循环:即使在恶劣环境的储存和取放后仍然保持完好无损且不卷翘,不会吸水受潮,即使解冻后也不发生撕裂。
Ultralow Temperature Pharmaceutical Label Adhesive

西氏的NovaGuard® SA Pro安全系统旨在保护医护人员和患者免受意外针刺伤害。NovaGuard® SA Pro安全系统具有防止操作过程中预激活的设计,仅需单手即可操作。该系统与ISO 0.5mL标准和1mL细长玻璃注射器(带针)兼容,其透明的外观便于药物检查和注射。此外,该装置的设计有助于玻璃预填充注射器的轻松组装,能够实现现有灌装设备轻松改装。
产品优势包括:
• 具有防止操作过程中预激活的设计
• 能轻松激活,便于终端用户使用
• 透明度高,便于药品检查和标签设置
• 易于在低速和高速灌装线上组装
• 与标准推杆兼容
• 防篡改功能,可防止硬护帽再次装上针头,符合EU指令2010/32/EU
West Pharmaceutical Services

阿普塔医药PremiumCoat®平台(包含产品和服务)为注射剂瓶和预灌封注射器提供溴化配方的ETFE覆膜瓶塞和活塞解决方案,以及为客户定制一整套关键数据和服务包,帮助客户加速药物研发。
PremiumCoat®解决方案可用于13mm和 20mm的注射剂瓶,1mlL预灌封注射器,以及正在商业化的1-3mL预灌封注射器。
阿普塔医药研发了一系列先进的检测评价方法来测试和体现PremiumCoat®的性能。阿普塔医药服务汇集了服务公司(阿普塔医药子公司)的专长,为客户提供端到端的服务,以加快产品研发并降低研发过程中的风险。

在制药市场中,假药问题日益严重。能够显示药品是否被开启过的安全封口标签可以帮助患者确认药品的真假和可靠性。我们的安全标签解决方案可以帮助您遵从法律法规要求并提升患者安全性。具体包括:
1.封口标签,移除后,会使包装或标签发生明显的、不可逆转的损坏或更改 - 不会增加包装的打开难度。
2.封口标签的功能包括纤维撕裂、VOID 效果、易碎纸张和薄膜以及全息图防伪。
3.存在验证解决方案,可以更轻松地确保防开启标签存在并正确粘贴在包装上。

1.可根据剂量要求进行客户定制化设计,有0.35ml,0.5ml,1ml,2.25ml。
2.现有的一次性注射器,设计原理都非常复杂,导致零部件繁多,普遍在15个以上,因此可靠性不高,装配难度大,而且产品成本高;我们提供了一种结构简单,零部件数量显著减少,且各注射步骤可靠性高的一次性自动注射器。
3.注射笔外壳体采用凸条设计,在满足防滑的同时不会造成手指或手掌的拿捏不适感。
4.采用新型的自动注射器触发装置,增加限位,避免二次触发,采用模块化配置,易于装配与使用,安装容错率较高,即便安装错误也可以快速进行替换,降低装配成本。
Suzhou Jiashu Medical Technology Co., Ltd.

1.通过在瓶盖中集成干燥剂,达到防潮作用,防止产品失效。
2.通过瓶与盖的配合达到密封效果,阻隔环境中的水气进入包装,延长包装的时效,同时具有反复密封作用,客户使用过程中依然能有效保护产品。
3.通过盖子的特殊设计,达到防儿童开启效果,兼具防盗功能。

1. 涂层工艺是用高纯度无溶剂气体。
2. 超纯单体,低碳环保。
3. 涂层材料使铝罐表面产生了一种低表面能具有疏水性,极大的提高了罐装物药物的传递率。

NovaPure®活塞的设计开发充分运用了“质量源于设计”(Quality by Design, QbD)这一原理。根据客户和终端用户的需求,西氏制定了全面的产品质量目标(QTPP)。始终将终端患者的需求放在首位,关键质量属性(CQA)的建立有助于确保整个生命周期内药品的质量、安全性和有效性。
NovaPure®活塞优化了启动力和滑动力,严格的设计质量标准减小了产品部件间的尺寸差异,适配不同注射体积和更高粘度范围的自动注射器的药物注射。
所有NovaPure®组件均符合西氏的严格质量标准:制药级清洗、灭菌、FluroTec®阻隔膜,以及完整的West Envision™自动外观检查。
West Pharmaceutical Services

1.创新性的压盖,匹配所有符合ISO标准的胶塞和西林瓶。
2.通过包装密封完整性验证(-80°C,常温,40°C)。
3.预装胶塞,免洗免灭。
4.无菌区同步加塞与压盖。
5.可手工压盖,无需任何工具。
6.无需轧盖工序,降低设备、公用设施、无菌区、人员、验证等投入。
7.无粘塞,不跳塞。
8.无铝屑,极大减少颗粒物。
9.可实现盖身喷码和贴标。
10.无金属边缘割破手套风险。

1. 该产品采用分体设计,盖子和管子是分开的且盖子及干燥剂一体,简化了客户端的流程,即不需要单独在管内投放袋装干燥剂或筒状干燥剂,避免了小孩误食和干燥剂污染产品。
2. 该包装由于是分体,很轻松地实现防盗环的功能,避免人为的开启后来投诉产品已被使用过的可能。
3. 设计简约美观,符合人体工学,盖子带水滴曲面的设计,可以轻易的单手开启,开启后盖子与管子相连,防止忘记合盖产品受潮。
4.该产品的最大的特点为:吸湿相关的功能优秀,对测试条的起到很好的保护。
提交企业:SANNER
 院外自我注射,操作简便,给药精准,高度智能、数据可追溯,提高病患依从性。适用范围广泛,可满足高粘度、大剂量药物注射要求(1mL, 2.25mL)。驱动装置重复使用,减少浪费,节约社会资源。
院外自我注射,操作简便,给药精准,高度智能、数据可追溯,提高病患依从性。适用范围广泛,可满足高粘度、大剂量药物注射要求(1mL, 2.25mL)。驱动装置重复使用,减少浪费,节约社会资源。

-2.png)
Target medicine types/ users:
Eye dropper/suitable to all users, especially friendly to senior, pediatrics and veterinary users.
Purpose of the R&D/ design/ improvement:
More accurate medication and improve medication compliance.
Product introduction:
Our new Risdrop™ product was carefully designed for the ophthalmic market, creating an innovative user-independent eye-dropper with a unique nozzle technology. The same size drop every time, helping reduce the risk of over dosage. The nozzle features a restricted flow channel insert allowing for a uniform drop size (variance +/-15%) regardless of container inclination and irrespective of squeeze force. The package helps the delivery of the drop to the right spot and is easy-to-hold and friendly to senior, pediatrics and veterinary users.
RISDROP™- User Independent Eye Dropper with Consistent Drug Dispensing
Berry Global Group, Inc.
.png)
Target medicine types/ users :
Aesthetic/mRNA vaccines/IV Infusion
Purpose of the R&D/ design/ improvement:
Innovation Polymer Syringe to meet high challenge drugs requirement.
Product introduction:
SCHOTT TOPPAC® is made of 100% high purity materials of Cyclic olefin copolymer (COC) which had good resistance to damage, transparency comparable to glass, and excellent barrier properties, no ion or metal released and no PH shift, highly suitable for biologics and low protein adsorption.
SCHOTT TOPPAC® Pre-Filled Syringe

Target medicine types/ users:
Applications for pills and capsules
* Over-the-counter (OTC)
* Ethicals
* Generics
* Nutraceuticals
* Veterinary medicine
Purpose of the R&D/ design/ improvement:
Designed to be Recyclable
Product introduction:
kpNext™ R1 is a PET mono rigid film that is designed to be fully recyclable within the RIC #1 stream. It is an alternative to vinyl blister films that will meet or exceed all your sustainability goals and work within your current manufacturing setup. Pharmaceutical companies and converters can utilize kpNext™ R1 on their existing form, fill and seal lines with no loss of line speed or a need to retool.

Target medicine types/ users:
Target medicines are asthma adn COPD drugs such as Budesonide Formoterol.
Purpose of the R&D/ design/ improvement:
PowdAir Plus has been designed to be a cost effective dry powder inhaler, made from four plastic components, and with no metal components. This minimises manufacturing and assembly costs. The sleek design ensures the device is easy to use for the patient.
Product introduction:
In developing markets, where demand for respiratory treatments is growing rapidly, there’s an increasing need to offer more choice to patients who use a dry powder inhaler (DPI).PowdAir Plus is a patented capsule-based DPI, ideal for these markets because of its advanced simplicity, ease of use and affordability.Most other DPIs have been designed for developed markets and incorporate multiple components and complex mechanics. In contrast, PowdAir Plus is a complete unit with no metal or separate parts. Neat and compact, its novel all-plastic, four-component design minimises manufacturing assembly and production costs, while improving the device’s resilience to frequent use.The sleek design of PowdAir Plus makes this inhaler very easy to use. It’s highly portable, and delivers an effective dose of medication with each capsule. Compatible with HPMC capsules in size 3, it can be used with any dry powder medicines.A particularly useful design feature is the way the device opens the capsule as the tray closes. Patients don’t need to pierce the capsule themselves, so using the inhaler is simpler, and theres no risk of getting the wrong dose.


Target medicine types/ users:
Compitable with dial-and-dose disposable pen(Unopen)
Purpose of the R&D/ design/ improvement:
Transforming the proven dial-and-dose pen platform into a connected
Product introduction:
Smart Pilot could Transform the proven dial-and-dose pen platform into a connected system though a single click (and clear visual feedback). Real-time tracking function not only provide dosing record for patient but also doctors and relation for better adherence and cure.
Smart Pilot™ -An Intelligent Device Designed for Mature Injection Pen Platforms
YPSOMED AG
.jpg)
Target medicine types/ users:
Applicable for sterile filling with vials
Purpose of the R&D/ design/ improvement:
Sustainable solution to treating the internal surfaces of pMDI canisters, and helping combat drug degradation/drug adhesion which are common problems within a pMDI, thus ensuring the patient receives the correct dose each and every time.
Product introduction:
The innovated elastomer sealing set can be widely used for serum products, Lyophilization, and IV solutions without filling in the different lines. This packing system can replace the general packing with vials, rubber stoppers and Aluminum seals, and simplifies production process and secured the products.
New Elastomer Sealing Solution includes special designed vials, plastic/elastomer sealing set, improving the current packing system without general metal material. In this case, no metal particles will be created. And for Lyophilization products, capping can be proceeded in Lyophilization machine. So that production process will be more efficiency and decreasing the cost.

Target medicine types/ users:
Vaccine and psychotropic drugs/drug manufacturers
Purpose of the R&D/ design/ improvement:
Improve the use experience and treatment effect, improve compliance
Product introduction:
Single dose trigger nasal spray device adopts a new structure with a spring as the driving force of drug delivery. Different users only need to press lightly to trigger the release force of the spring, which can push the storage component to move and make the medium in the storage component spray to the nasal cavity through the nozzle component. It can be applied to different populations and is easy for patients to operate, and ensures that different types of patients can deliver the same dose at the same injection speed, thus improving the use experience, treatment effect and compliance.
Aerosol – Single Dose Trigger Nasal Spray Device

Target medicine types/ users:
Pharmaceutical packaging products
Purpose of the R&D/ design/ improvement:
Pharmaceutical packaging that requires higher storage time and is more sensitive to moisture
Product introduction:
This product is optimized in the structure of cold forming process aluminum by processing and compounding of key moisture-absorbing materials. The structure is OPA/AL/polyolefin moisture-absorbing layer/PVC, which takes into account the overall cost of moisture absorption, especially the cost of PTP. PTP can still use ordinary PTP with registration number. It can greatly reduce the cost of use and R&D for customers. At the same time, our company adopts multi-layer co-extrusion technology in the polyolefin moisture-absorbing layer and adds a barrier layer and slow-release layer. We can design and adjust the formula according to the customers’ requirements of the moisture absorption curve (The relationship between time and moisture absorption) to achieve the packaging effect matching with the drug compatibility.
Moisture Control Functional Cold Forming Process Aluminum Packaging Products Mainly for The Type of Drugs

Target medicine types/ users:
Solid preparations, injections, ointments, etc.
Purpose of the R&D/ design/ improvement:
Long-term improvement of efficiency and continuous cost saving.
Product introduction:
The project improved brand consistency management standards, increased management and production efficiency, reduced production and transportation costs, and achieved the long-term sustainable goal of effectively improving efficiency and saving costs.

Target medicine types/ users:
an oral liquid preparation package for accurate quantitative
Purpose of the R&D/ design/ improvement:
accurate quantitative and non-cleaning
Product introduction:
The utility model relates to an oral liquid preparation package for accurate quantitative and non cleaning. The storage part of the taking measuring cup is used to store the poured liquid preparation, and a scale line is arranged inside to accurately measure and quantify the taking amount. The taking part extends out of the storage part, and the liquid preparation in the storage part is guided into the patient's mouth from the accurately measured taking part, which makes it more convenient to take and avoids spilling the liquid preparation during taking. The built-in taking part does not contact the preparation after use, and is isolated from the air after locking the bottle cap, which does not affect the drug quality. At the same time, it reduces the inconvenience of patients' use and cleaning caused by external measuring cup.
The Utility Model Relates to An Oral Liquid Preparation Package for Accurate Quantitative and Non-Cleaning.

The Judges in 2022

World Packaging Organisation
President





Mr. Zhu Gui Lin

Mr. Zhu Wei Ming






Previous Partners



Fill in the form to enter the Innopack Awards China 2024
CONTACT US |
![]() +86 21 3339 2255
+86 21 3339 2255
![]() Iris.Feng@imsinoexpo.com
Iris.Feng@imsinoexpo.com
![]() +86 21 3339 2436
+86 21 3339 2436
![]() Wayne.Zou@imsinoexpo.com
Wayne.Zou@imsinoexpo.com

